The Kumar Lab received a grant from NIDA to build new models of addiction. The press release is here
https://www.jax.org/news-and-insights/2020/july/building-better-mouse-models-for-addiction-research
The project summary is available here:
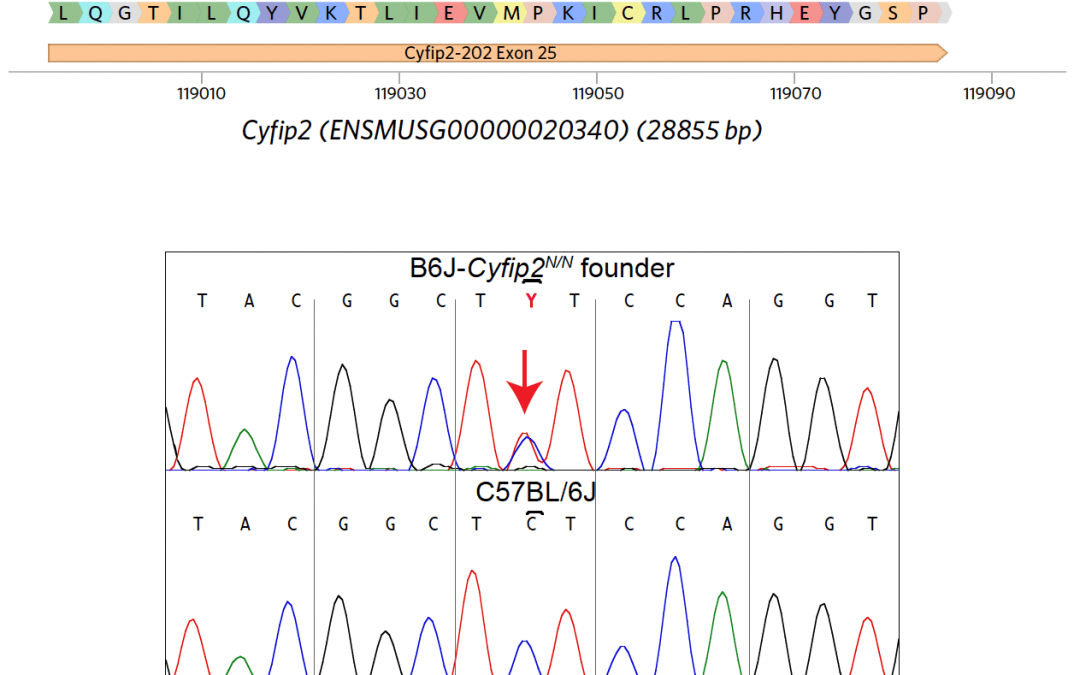
PROJECT SUMMARY Addiction is an enormous economic, personal, and social burden, costing over $600 billion per year in the U.S. Understanding vulnerability to addiction, and developing effective therapies, requires identifying the genes and pathways that mediate the addiction process. Our long-term goal is to develop novel genetic models for addiction-relevant phenotypes, and use these models to characterize the genetic mechanisms of addiction. Here, we propose to extend our previous work that led to the cloning of a QTL that regulates addiction and the subsequent identification of Cyfip2 in mouse substrains as a regulator of cocaine acute and sensitized responses. We and others have since shown that this mutation regulates food reward, nicotine preference, and alcohol preference (preliminary data). In addition, we have shown that Cyfip2 regulates voluntary self- administration of cocaine in the IVSA assay, the gold standard in the addiction field. Cyfip2 is a hub for signal integration from multiple pathways, including the small GTPase RAC1, WIRS domain receptors, and Fragile X family signaling. We hypothesize that this signal integration by Cyfip2 is critical for reward behaviors. In response to PAR-19-278, we now propose to use precise genome engineering in mice to generate and functionally validate 5 variants in Cyfip2 (1-2 amino acid substitutions each) that specifically perturb each of these signal integration events. These mutations are designed using published biochemical data and in consultation with our Co- Investigator Dr. Chen, who is a leader in Cyfip biophysics and structure. In the R21 phase (Aim 1), we will leverage the mouse genetics expertise of the Jackson Laboratory to generate by CRISPR/Cas9 a set of 5 Cyfip2 signaling mutants. Specific milestones for progression to the R33 phase are (i) viability of the mutants, since the knockout of Cyfip2 is postnatal lethal, and (ii) the lack of functional off-target edits. We will then characterize these mutants comprehensively for cocaine and natural reward behavior (R33, Aim 2). To gain insight into mechanisms underlying these behaviors, we will determine the biochemical interactome of each mutant in mouse brain regions using a comprehensive mass spectrometry-based study (R33, Aim 3). The successful completion of this project will yield 5 preclinical mouse models of addiction transition for the scientific community, as well as information about specific signaling pathways that are critical for transition to addiction and that can be targeted for therapy.